Strontian Process on:
[Wikipedia]
[Google]
[Amazon]
The strontian process is an obsolete chemical method to recover sugar from molasses. Its use in Europe peaked in the middle of the 19th century. The name ''strontian'' comes from the Scottish village
 Strontium carbonate is a recycled coreactant in this process.
# Strontium carbonate is
Strontium carbonate is a recycled coreactant in this process.
# Strontium carbonate is
(PDF; 4,3 MB)
''(German).'' One of the biggest mines, at
(PDF; 6,5 MB)
''In German.'' * Heriot, T. H. P.: ''The Manufacture of Sugar from the Cane and Beet'', Green and Company, 1920, pp. 341–34
(archive online)
* ''Krause, G.: ''Der Schiedsspruch in Sachen des Scheibler'schen Monostrontiumsaccharat-Patentes'', Chemiker Zeitung, nr. 32, 19th April, 1885
(PDF; 4,94 MB)
''In German.''
Strontian
Strontian (;
gd, Sròn an t-Sìthein) is the main village in Sunart, an area in western Lochaber, Highland, Scotland, on the A861 road. Prior to 1975 it was part of Argyllshire. It lies on the north shore of Loch Sunart, close to the head of ...
where the source mineral strontianite (strontium carbonate
Strontium carbonate (SrCO3) is the carbonate salt of strontium that has the appearance of a white or grey powder. It occurs in nature as the mineral strontianite.
Chemical properties
Strontium carbonate is a white, odorless, tasteless powder. B ...
) was first found.
Chemistry
 Strontium carbonate is a recycled coreactant in this process.
# Strontium carbonate is
Strontium carbonate is a recycled coreactant in this process.
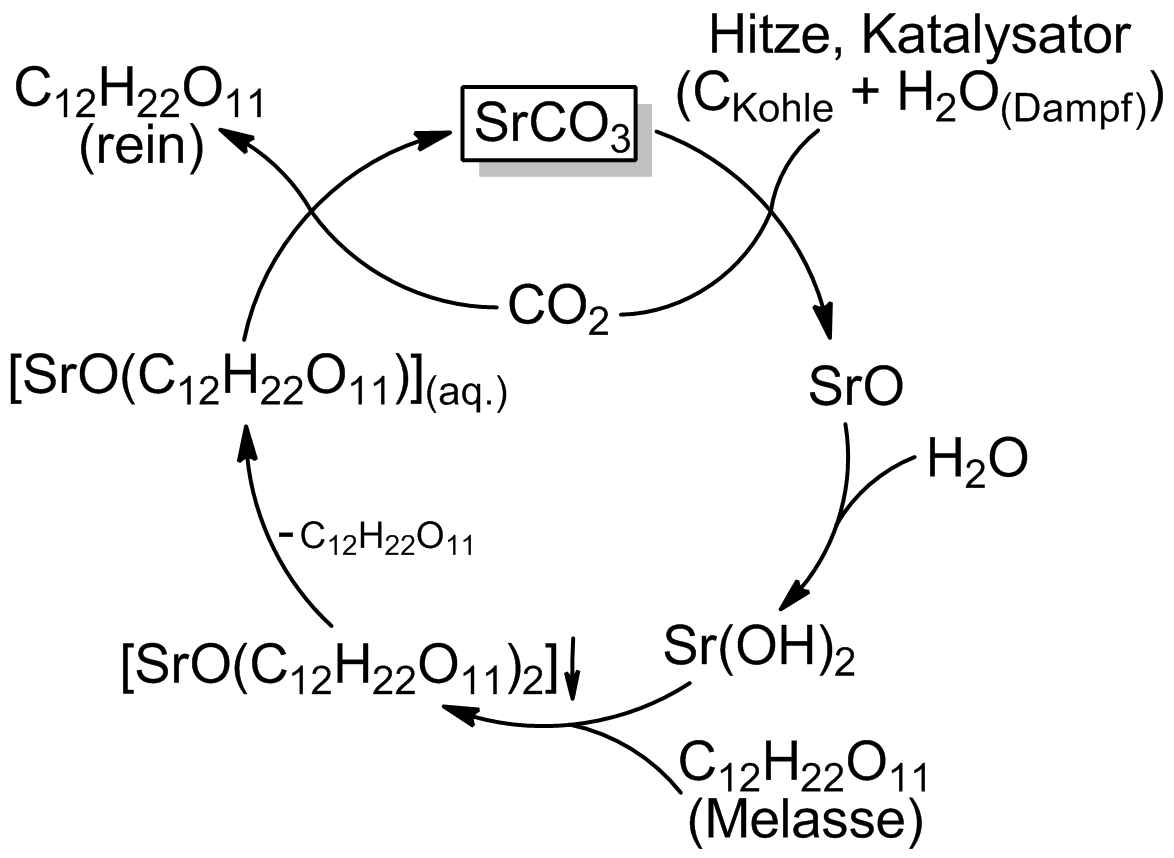
# Strontium carbonate is calcined
Calcination refers to thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), gener ...
with carbon in the presence of steam to form strontium hydroxide. The strontium and carbon dioxide formed are rejoined later in the process, forming strontium carbonate once again.
#: SrCO3 + C + H2O = Sr(OH)2 + 2 CO2
# In a molasses solution kept near 100 °C, the hydroxide reacts with soluble sugars to form water and the poorly soluble strontium saccharide which is filtered out, but kept awash in near-boiling water.
#: Sr(OH)2 + 2C12H22O11 = SrO(C12H22O11)2 + H2O
# The saccharate liquid is cooled to 10 °C, cracking off one of the sugars
#: SrO(C12H22O11)2 = SrO(C12H22O11) + C12H22O11
# The carbon dioxide (from the calcination) is bubbled through the saccharate solution, cracking off the second sugar and reforming the strontium carbonate, which is filtered off.
#: SrO(C12H22O11) + CO2 = SrCO3 + C12H22O11
# The sugar is then extracted through evaporating the remaining solution.
There are two types of strontium saccharide
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ma ...
: one at low temperature, the strontium monosaccharide; and the second at high temperature, the strontium disaccharide
A disaccharide (also called a double sugar or ''biose'') is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, la ...
.
History
Molasses is the first stage output of several differentsugar production
The sugar industry subsumes the production, processing and marketing of sugars (mostly sucrose and fructose). Globally, most sugar is extracted from sugar cane (~80% predominantly in the tropics) and sugar beet (~ 20%, mostly in temperate climate ...
processes, and contains more than 50% sugar. The French chemists Hippolyte Leplay and Augustin-Pierre Dubrunfaut developed a process for extracting sugar from molasses, reacting them with barium oxide
Barium oxide, also known as baria, is a white hygroscopic non-flammable compound with the formula BaO. It has a cubic structure and is used in cathode ray tubes, crown glass, and catalysts. It is harmful to human skin and if swallowed in larg ...
, to give the insoluble barium-saccharates. In 1849, they expanded their patent to include strontium salts. Apparently, this patent application had the only purpose to legally secure the so-called ''baryte process'', since the strontian process from Leplay and Dubrunfaut probably wouldn't work as described.
Only later, through the works of Carl Scheibler, was it possible to apply the strontian process in an industrial basis.
According to Scheibler the procedure must be carried out at boiling temperatures.
Repercussion in Germany
The Scheibler procedure came into use in the Dessauer Sugar Refinery (in Dessau), through Emil Fleischer. In the Münsterland region, its arrival caused a ″gold fever″ breakout, regarding the strontianite mining.Martin Börnchen: ''Der Strontianitbergbau im Münsterland'(PDF; 4,3 MB)
''(German).'' One of the biggest mines, at
Drensteinfurt
Drensteinfurt (in low German ''Stewwert'') is a town in the district of Warendorf, in North Rhine-Westphalia, Germany. It is situated approximately 15 km north of Hamm and 20 km south of Münster. The villages Rinkerode in the north an ...
, was named after Dr. Reichardt, the director of the Dessauer Sugar Refinery. A further place the strontian process came to be used was the Sugar Factory Rositz (in Rositz
Rositz is a municipality in the district Altenburger Land, in Thuringia, Germany.
History
Within the German Empire (1871-1918), Rositz was part of the Duchy of Saxe-Altenburg.
An RAF raid bombed the oil refinery in Rositz on February 14/15, 194 ...
).
Yet by 1883, the demand for strontianite had begun to shrink. First, it was replaced by another strontium mineral ( celestine), that could be imported from England, in a cheaper way. Second, the prices for sugar decreased so much, that the production from molasses was no longer worthwhile.
Literature (further reading)
* Börnchen, Martin : ''Strontianit'', Exhibition guide from the University Library of theFree University of Berlin
The Free University of Berlin (, often abbreviated as FU Berlin or simply FU) is a public research university in Berlin, Germany. It is consistently ranked among Germany's best universities, with particular strengths in political science and t ...
, 2005''(PDF; 6,5 MB)
''In German.'' * Heriot, T. H. P.: ''The Manufacture of Sugar from the Cane and Beet'', Green and Company, 1920, pp. 341–34
(archive online)
* ''Krause, G.: ''Der Schiedsspruch in Sachen des Scheibler'schen Monostrontiumsaccharat-Patentes'', Chemiker Zeitung, nr. 32, 19th April, 1885
(PDF; 4,94 MB)
''In German.''
References
{{reflist Chemical processes Industrial processes Catalysis Strontium Strontium minerals